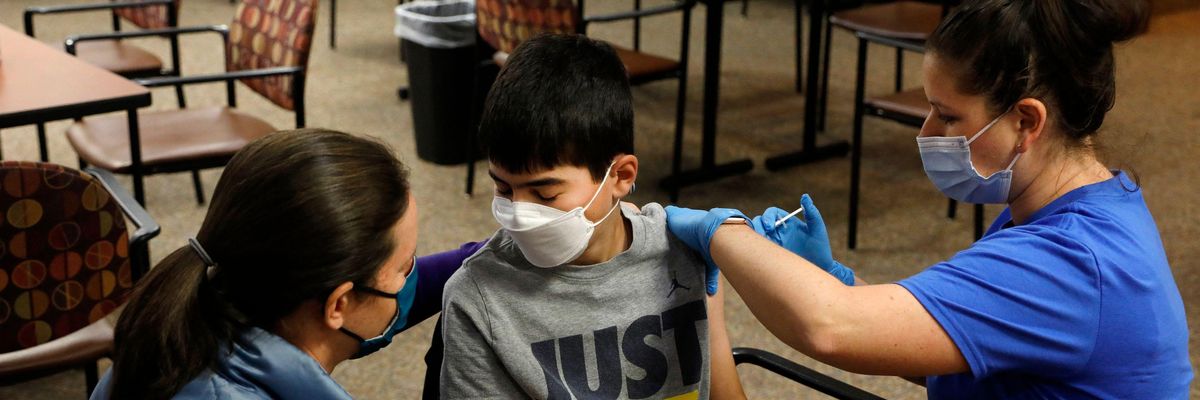
The Food and Drug Administration's independent advisory panel on vaccine safety unanimously approved the Pfizer-BioNTech Covid-19 vaccine for children ages 5 to 11 on Tuesday, paving the way for nearly all school-aged students in the U.S. to have protections from the deadly virus available to them in the coming weeks and months.
With just one abstention, the advisory panel voted 17-0 to approve the vaccine for younger children following a thorough review of data provided by the drug makers and outside review.
According to the Associated Press:
The FDA isn't bound by the panel's recommendation and is expected to make its own decision within days.
If the FDA authorizes the kid-size doses, there's still another step: Next week, the Centers for Disease Control and Prevention will have to decide whether to recommend the shots and which youngsters should get them.
"An FDA staff analysis released late Friday suggested the product's benefits outweigh the risks of adverse side effects to kids," reports Politico. "However, the document also noted that balance could change if Covid case rates again fell to those seen in June, depending on the extent to which instances of myocarditis--an inflammatory heart condition linked to messenger RNA vaccines--occur in that age group."
NPRreports:
The agency typically goes along with the advice of its expert panels, though it isn't bound to do so. It will issue a decision within the next several days. If the FDA authorizes the vaccine for younger children, as seems likely, another panel of experts advising the Centers for Disease Control and Prevention age group would make recommendations on its use next week.
The vaccine provides a broad defense against COVID-19 and "effectively neutralized the delta variant" in this age group, said Dr. William Gruber, senior vice president of vaccine clinical research and development at Pfizer, speaking during the committee hearing.
A dose of Pfizer for young children contains one-third the amount of active ingredient as the adult dose. Children would receive a second dose 21 days or more after their first shot.
Gruber said the dose size was chosen to "strike the right balance" between providing strong immunity and limiting side effects. He said that the observed adverse effects seen in the company's studies "did not suggest any safety concerns." An FDA review supported that conclusion.
Last week, following a White House rollout, the American Academy of Pediatrics (AAP), welcomed the Biden administration's plan for rolling out vaccines to the nation's children once FDA approval was set.
"This is a key moment in our nation's response to the COVID-19 pandemic," said in a statement on Oct. 20. "More than 6 million children have been diagnosed with COVID-19, including 135,000 just last week. So far, not all children have had access to vaccines but we are hopeful that very soon, school-aged children will benefit from the life-saving protection of the COVID-19 vaccine."